Hydrogen fuel cells are not directly batteries, since they do not store energy, but rather continuously produce it.
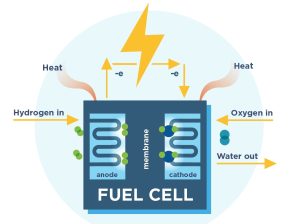
Hydrogen fuel cells are not directly batteries, since they do not store energy, but rather continuously produce it. They convert chemical energy into electrical energy by a reaction between Hydrogen and Oxygen (fig. 1).

Fig.1: The fuel cell
https://www.fchea.org/h2-day-2019-events-activities/2019/8/1/fuel-cell-amp-hydrogen-energy-basics
The products of this reaction are water and heat which makes it environmentally friendly compared to other ways of producing electricity. For example, fossil fuels as the fuel for the reaction, emit lots of carbon dioxide. Fuel cells can produce more energy than combustion engines or steam turbines or other technologies that produce electricity, since the chemical energy gets directly turned into electrical energy without losing any of it during the process, and the heat and water that are products of the reaction can be used for heating or cooling applications.
A hydrogen fuel cell has an efficiency of about 60% at the moment, however, with further research this can be increased even further to make those systems more efficient. For comparison: electrolysis has an efficiency of around 60% to 70% and modern petrol or gasoline engines reach an efficiency of about 24%. They do not need to be recharged, the only thing needed is a steady flow of fuel as a source for the reaction with oxygen. Besides that fuel cells are also scalable, which means multiple fuel cells can be combined into a larger system, which makes them suitable for small scale operations like a car, but also just as useful for powering an entire utility grid. If combined with a storage system and recycling of hydrogen, it makes them entirely pollution-free.
Hydrogen fuel cells have another major advantage over other types of energy production: they are really easy for transportation since there are no loose parts inside the fuel cell which might break and since they are scalable to any sizing. Smaller fuel cells, weighing around 10 kilograms, can produce up to 5 kilowatts for remote locations or up to 50 watts for powering smaller devices. A fuel cell is composed of an anode, cathode, and an electrolyte membrane (fig. 1). A typical fuel cell works by passing hydrogen through the anode of a fuel cell and oxygen through the cathode. At the anode site, a catalyst splits the hydrogen molecules into electrons and protons. The protons pass through the porous electrolyte membrane, while the electrons are forced through a circuit, generating an electric current and excess heat. At the cathode, the protons, electrons, and oxygen combine to produce water molecules. As there are no moving parts, fuel cells operate silently and with extremely high reliability.
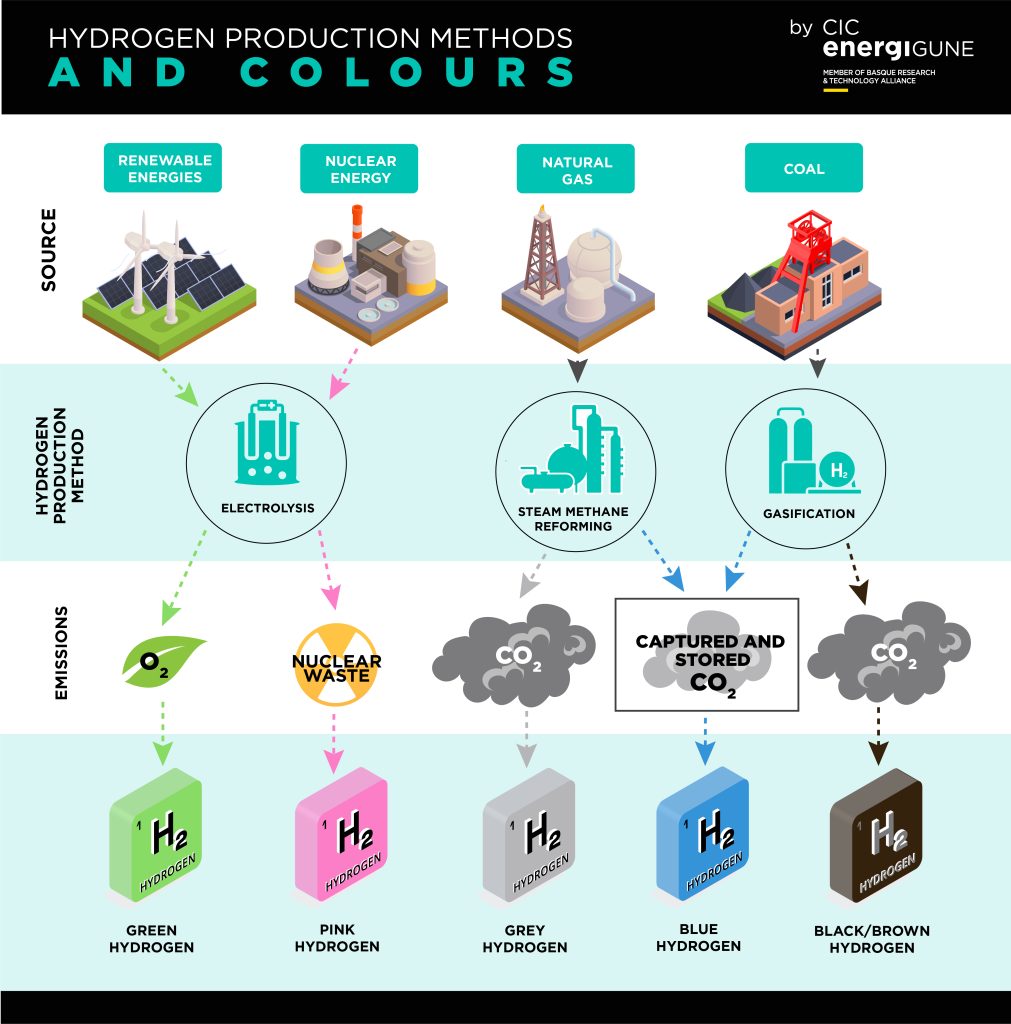
Fig. 2: The hydrogen production methods
https://cicenergigune.com/en/blog/hydrogen-production-methods-colours
Are hydrogen fuel cells our future?
Science is still researching a way to make hydrogen fuel cells available for wider use. One of the main problems is the cost of making those fuel cells. They require platinum as one of the largest component materials for the catalyser, however researchers are working hard on finding a fuel cell design which uses a non-platinum catalyst approach to make it cheaper and easier to produce. Another big issue which is still being worked on is the extraction of hydrogen. This process takes a lot of energy to achieve and mostly fossil fuels are used for that process of extraction which counterfeits the green label of hydrogen engines at the moment, however clean ways of producing hydrogen have been found but are not yet widely used (fig. 2).
The next issue is that the energy infrastructure in almost all countries is not designed for an immense use of fuel cells as the main source of energy, so a lot of the infrastructure that relies on energy, including vehicles, would have to be retrofitted.
The last but also least important downside which comes with hydrogen fuel cells is the flammable nature of hydrogen. This problem, however, has been dealt with a lot in the industry already, for example by certain ways of storage and safety features. Especially hydrogen cars have implemented those safety features very successfully making those cars as safe if not even safer than cars fuelled by other types of energy like combustion or electricity.
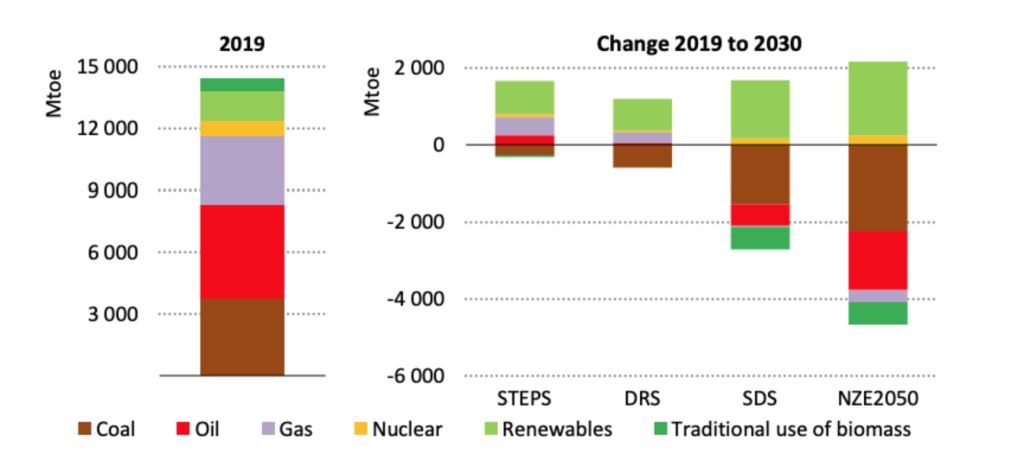
Fig. 3: The statistics about global production of hydrogen by colour. More=million tonnes of oil equivalent. (Source: IEA World Energy Outlook 2020).
Hydrogen fuel cells have existed for almost 2 centuries. We use them currently to power the electrical systems on spacecraft and to supply electricity on earth, also they are designed to last the lifetime of the vehicle.
Hydrogen itself is the cleanest source of energy there is available for humanity, and hydrogen fuel cells have proven higher efficiencies in generating energy compared to any other technology that is used in energy production. If research goes on, cleaner ways of producing hydrogen become more widely used and the infrastructure for it gets built, hydrogen fuel cells can become the future not only for vehicles or electronic devices, but of the entire worldwide energy production. Looking at the accomplishments scientists have made over the last decades it is almost certain that hydrogen fuel cells will play a major role in energy production in the future.
Jonas Behr
References:
https://www.twi-global.com/technical-knowledge/faqs/what-is-a-hydrogen-fuel-cell
https://www.fchea.org/fuelcells
https://www.tuev-nord.de/en/company/energy/hydrogen/hydrogen-fuel-cell